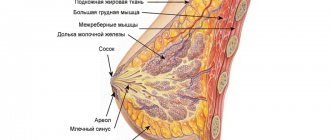
Ultrasound of the mammary glands with implants is the main non-invasive method used to diagnose common pathologies of the mammary gland. An ultrasound should be done immediately before plastic surgery, and then repeated annually after it. The technique allows you to observe the structures of the organ after surgery, as well as timely diagnose various complications, inflammation, and neoplasms.
Also, during a diagnostic study, the diagnostician assesses the condition of the implant, determines its position and integrity. At the Hemostasis International Clinic, ultrasound examination of mammary glands with implants is carried out using modern, highly sensitive equipment that can detect any, even minor, pathological change, which is very important for early diagnosis and timely therapy.
Features of the procedure
Examination of the mammary glands after mammoplasty is carried out for two purposes:
- Assess breast tissue and the condition of regional lymph nodes.
- Check the condition of the implants and the body’s reaction to them.
Ultrasound examination after implantation is recommended annually. This is a safe, reliable, painless diagnostic method that allows you to monitor the condition of the organ and detect any pathological changes in the initial stages of development.
No special preparation is required for the procedure. The study is carried out from 6 to 12 days of the menstrual cycle. Ultrasound examination after mammoplasty can be planned or emergency. A routine examination is recommended to be performed a week, a month, six months after surgery. Then you need to visit a mammologist and have an ultrasound scan every year. If cysts, fibromas, or focal neoplasms are identified during diagnosis, re-diagnosis is prescribed after 6 months. An emergency ultrasound of the mammary glands with implants is performed when the patient’s well-being deteriorates, damage to the prosthesis is suspected, or the formation of tumors of various etiologies.
Ultrasound examination after mammoplasty has its own characteristics. When examining breast glands with prostheses, it is necessary to take into account that implants are installed in the lower segments, so the gland tissues are shifted upward and flattened. In addition, it is important to find out where the prosthesis is installed - under the gland or under the muscle.
Ultrasound in the first days after implantation evaluates the presence or absence of fluid around the prosthesis, which helps to exclude gray matter and hematoma, and if they are detected, promptly prescribe treatment. It is also important to evaluate the shape, outline, and integrity of the implant shell.
In the later stages after mammoplasty, ultrasound is used to assess the condition of the prosthesis itself, its shape, contours, mobility, and the presence of pericapsular fibrous changes. Particular attention is paid to neoplasms in the mammary glands, because any tumor can degenerate into malignant in the future. The condition of regional lymph nodes is carefully studied. The conclusion is made on the basis of ultrasound examination, examination, palpation, and laboratory diagnostics. If an ultrasound reveals a tumor with signs of malignancy, an additional MRI of the breast is prescribed.
3D ultrasound after mammoplasty
Ultrasound machine RS85
Revolutionary changes in expert diagnostics.
Impeccable image quality, lightning-fast operating speed, a new generation of visualization technologies and quantitative analysis of ultrasound scanning data.
Introduction
The problem of breast enlargement has worried women since ancient times. According to statistics, the number of people wishing to restore lost tissue volume or compensate for natural deficiencies with the help of plastic surgery increases annually by 10% [1].
The history of surgical breast augmentation goes back more than 100 years. The introduction of liquid paraffin, wax, and vegetable oils into the mammary gland was accompanied by suppuration, the appearance of compactions, rejection of foreign material, the formation of fistulas and granulomas. Foreign materials caused embolism of blood vessels and accumulated in the organs of the reticuloendothelial system. They tried to make prostheses from glass, metal, and ivory, but they also often shifted and were rejected.
In 1895, surgeon Vincenzo Cerni was the first to replace a defect with a lipoma removed from the same patient after excision of part of the mammary gland.
Since the 40s of the last century, silicone has been used as an injection material. The first women to be injected with liquid silicone to enlarge their mammary glands were Japanese prostitutes, since after World War II the American military contingent on the Japanese islands had a demand for women with full breasts. The consequences were the same: fibrosis, necrosis of soft tissues, infection, fistulas, granulomas, migration of the substance into the surrounding tissues with subsequent sclerosis.
In the early 60s, prosthetics were created from polyvinyl alcohol in the form of a sponge. The results became better, but rough connective tissue also formed around the sponge, which deformed the prosthesis; it became unnaturally round, hard and decreased in volume.
At the same time, the first silicone shell endoprostheses filled with gel or saline were implanted.
In the 90s, injection plastic surgery was again attempted with new polyacrylamide hydrogel water-swelling materials similar to human adipose tissue. But the same complications began to arise again and the toxicity of the gel was proven. Injection plastic surgery continues to occupy the minds of surgeons, and 100 years after Cherny’s experience, the technique of lipotransfer or lipofilling appears: an increase of 1–1.5 sizes by introducing into each breast about 300 ml of own fat obtained by liposuction, that is, by washing out with a special solution under low pressure.
Modern shell endoprostheses have a dense capsule of 2–3–4 or even 5 layers of seamless silicone elastic rubber, filled with gel, or less often, saline filler [1]. The shape can be spherical or teardrop-shaped, the surface smooth or textured. The prosthesis can be placed under the glandular-fibrous complex or under the pectoralis major muscle. But even when installing shell endoprostheses, seromas, hematomas, capsular fibrosis and capsular contracture, implant ruptures, gel migration, inflammation, and liponecrosis can occur. 92% of complications occur in the late postoperative period – over 2 months.
Currently, ultrasound examination (US) is one of the main methods for diagnosing diseases of the mammary glands; the information content of the study increases with increasing scanning frequency (5.0–7.5–10.0 MHz) and the size of the working surface of the sensor 40–60 mm. Thus, for radial ductal echography, a sensor with a frequency of up to 13 MHz and a length of 92 mm is used [2].
The most modern technique that allows you to obtain the most complete image of the mammary gland is automatic volumetric scanning, or ultrasound tomosynthesis, sonotomography [3] using a special attachment that provides automatic movement of a high-frequency sensor (15 MHz) 15.4 cm wide, which registers in one pass 318 slices 0.5 mm thick. The maximum volume of tissue for research is 1552.3 cm3 [4]. The obtained data is analyzed on a workstation, saving the entire data array provides the possibility of post-processing, and standard installations ensure high reproducibility of the technique.
Types of breast structure
- ASR 1 (type A) – predominance of adipose tissue.
- ASR 2 (type B) – the glandular-fibrous complex occupies less than 50%.
- ASR 3 (type C) – glandular-fibrous complex occupies more than 50%.
- ASR 4 (type D) – the mammary gland consists 100% of the glandular-fibrous complex, “dense background”.
Normally, the reconstructed coronal section (Fig. 1a) reflects the radial structure of the mammary gland, resembling images obtained with tomosynthesis (Fig. 1b).
Rice. 1.
Normal types of mammary gland structure, comparison of results of automatic volumetric scanning and tomosynthesis.
A)
Automatic volumetric scanning, coronal section.
b)
Tomosynthesis.
Today, in his practical work, an ultrasound diagnostic doctor may encounter the consequences not only of injection or sheath mammoplasty, performed for purely cosmetic purposes, but also with the results of restoring the shape and volume of the mammary gland after surgical treatment of a tumor, including in combination with radiation therapy. As illustrations, we present the following clinical observations in which automatic ultrasound scanning of the mammary glands was performed on a modern ultrasound device with an ABVS (automatic breast volume scanning) attachment.
Clinical observation 1
Patient A., 45 years old, complained of painful lumps in both mammary glands. From the anamnesis it is known that 15 years ago injection mammoplasty was performed - the introduction of gel into both mammary glands in order to increase volume. With automatic volumetric scanning (Fig. 2a, 2b), as well as with tomosynthesis (Fig. 2c, 2d), the gel is detected in the form of scar-gel conglomerates with clear contours; encapsulated masses and diffuse impregnation of tissues. Over 4 years of observation, the ultrasound picture did not change. Conclusion: polyacrylamide mammary syndrome.
Rice. 2.
Results of examination of the mammary glands of patient A., polyacrylamide mammary syndrome.
A)
Automatic volumetric scanning of the right breast.
b)
Automatic volumetric scanning of the left breast.
V)
Tomosynthesis of the right breast.
G)
Tomosynthesis of the left breast.
Obviously, the presence of gel in breast tissue makes it difficult to diagnose other diseases; in this situation, a multiparametric approach and dynamic observation are certainly necessary [5].
It is believed that after injection of your own fat, taken from the abdominal area, up to 75% of it remains viable and is included in the metabolism, the rest of the fat is absorbed.
Clinical observation 2
Patient P., 36 years old. He makes no complaints. From the anamnesis it is known that 2.5 years ago, lipotransfer was performed to correct the volume of the mammary glands. During mammography, diffusely scattered amorphous calcifications of various sizes with a tendency to grouping are noted in the mammary glands. With automatic volumetric scanning (Fig. 3a, 3b), multiple areas of increased echogenicity with liquid inclusions with partially calcified walls are visualized in the subcutaneous fat tissue - cysts formed in areas of liponecrosis. With tomosynthesis (Fig. 3c, 3d), these areas are visualized as multiple intradermal calcifications.
Rice. 3.
Results of examination of the mammary glands of patient P., 2.5 years after lipotransfer.
A)
Automatic volumetric scanning, coronal section.
b)
Automatic volumetric scanning, sagittal section, enlarged fragment.
V)
Tomosynthesis of the right breast.
G)
Tomosynthesis of the left breast.
Since the introduction of the latissimus dorsi musculocutaneous flap into the range of reconstructive materials in the late 1970s, its use has become popular in reconstructive plastic surgery for patients with breast cancer [6].
Clinical observation 3
Patient V., 70 years old. Observed for 18 years after radical resection of the left breast for stage I cancer with simultaneous plastic surgery of the latissimus dorsi muscle. With automatic volumetric scanning of the left breast in the medial projection in a coronal section at the skin level, as in a regular photograph, the area of the postoperative scar is clearly visible, stretching from the lower internal through the upper internal and entire upper external quadrant, the mammary gland is deformed, the nipple is preserved (Fig. 4a). At a depth of 19.5 mm (Fig. 4b), the postoperative scar is still visible, below the nipple, marked with a yellow marker, there is a shadow of calcification, which has an uneven but smooth contour, and at the border of the outer quadrants there is a large hypoechoic area with an uneven contour without a symptom radiance, in the cine-loop mode it is clear that this is a muscle flap that stretches from the back.
Rice. 4.
Results of automatic volumetric scanning of the left breast of patient V., condition after radical resection and plastic surgery with the latissimus dorsi muscle.
A)
Coronal section at a depth of 0 mm.
b)
Coronal section at a depth of 19.5 mm.
V)
Horizontal cut.
Clinical observation 4
Patient M., 73 years old, complains of periodic discomfort in the right mammary gland. From the anamnesis it is known that in May 2014 she underwent a radical resection of the right breast with simultaneous nipple plastic surgery for stage I cancer (histologically - infiltrative ductal carcinoma 1.0 x 0.8 cm, RE-8; RP-0; HER- 2; neu–0; Ki 67–25%) followed by external beam radiation therapy at 50 Gy. Hormone therapy with tamoxifen 20 mg daily has been carried out since September 2014. Since the patient has macromastia, the volume of removed tissue was not replenished. Upon examination in June 2014, the right mammary gland was deformed due to surgery, swollen, hyperpigmentation was observed on the skin of the radiation fields, and diffuse thickening of soft tissues was observed in the area of the scar.
With automatic volumetric scanning (Fig. 5), there is a thickening and diffuse increase in the echogenicity of the skin, the picture of the surface section resembles a lemon peel (Fig. 5a), the echogenicity of subcutaneous fat is increased (Fig. 5b), and the lymphatic vessels are significantly dilated (Fig. 5c) .
Rice. 5.
Results of automatic volumetric scanning of patient M., 1 month after the end of treatment (radical resection of the right breast with simultaneous nipple plastic surgery and radiation therapy).
A)
Thickening and diffuse increase in echogenicity of the skin, the picture of the surface section resembles a lemon peel.
b)
Increased echogenicity of subcutaneous fat.
V)
Lymphatic vessels are significantly dilated.
After 3 years, in 2020 (Fig. 6): the lemon peel symptom is no longer detectable (Fig. 6a), there is no swelling of the fiber (Fig. 6b), there is no dilation of the lymphatic vessels (Fig. 6c). However, postoperative scars are clearly visible throughout the entire depth of the tissue.
Rice. 6.
Results of automatic volumetric scanning of patient M., 3 years after the end of treatment (radical resection of the right breast with simultaneous nipple plastic surgery and radiation therapy).
A)
The lemon peel symptom is not detected.
b)
There is no swelling of the fiber.
V)
There is no dilation of lymphatic vessels.
The ultrasound picture of an unchanged shell implant during automatic volumetric scanning (Fig. 7) is characterized by smooth contours, a continuous capsule and homogeneous contents. A fold depth of up to 4 mm is considered acceptable. The thickness of the periprosthetic fibrous capsule should not exceed 1.5 mm.
Rice. 7.
Ultrasound image of an unchanged two-layer smooth capsule implant of the left breast.
A)
Coronal section.
b)
Sagittal section.
Clinical observation 5
Patient P., 50 years old, came in 4 years after endoprosthetics with complaints of periodic discomfort in the left mammary gland. With automatic volumetric scanning, the unevenness of the anterior surface of the right breast implant is clearly visible (Fig. 8a), and it is easy to assess the number and extent of folds. In the left mammary gland (Fig. 8b), the glandular-fibrous complex is shifted to the upper outer quadrant, where several small cysts are visualized.
Rice. 8.
Results of automatic volumetric scanning of the mammary glands of patient P., 4 years after endoprosthetics.
A)
Right mammary gland, anterior projection, coronal section.
b)
Left breast, lateral view, coronal section.
The image of textured implants differs from smooth capsule implants in the granularity of the structure of their own capsules (Fig. 9a, b). With the same depth of folds (4 mm each), the contents may be different: in Fig. 9a, in the contents of the fold, small hyperechoic inclusions without shadows are clearly differentiated, which is a sign of inflammation, in Fig. 9b the contents are homogeneous. There is one more difference: in Fig. 9a, the periprosthetic fibrous capsule is stretched over the fold like a bowstring [3], while in Fig. 9b, the fibrous capsule is of uneven thickness, but sags above the fold, that is, it has not yet wrinkled. Thus, in Fig. 9a – initial phenomena of capsular contracture; in Fig. 9b – capsular fibrosis.
Rice. 9.
Textured implant.
A)
Initial phenomena of capsular contracture.
b)
Capsular fibrosis.
Clinical observation 6
Patient K., 41 years old, came in 12 years after breast surgery with complaints of deformation of the left mammary gland. On mammograms of the left breast, a retromammary shadow of the implant with clear and even contours, diffuse fibrocystic mastopathy predominantly with a glandular component and signs of adenosis, and well-defined glandular tissue are determined. Against this background, it is not possible to judge the presence of nodular formations (dense background). With automatic volumetric scanning in the left mammary gland, a smooth-capsular two-layer implant with a pronounced deformation along the anterior surface due to a bizarre pattern of folds, a hypoechoic area is visualized above the nipple (Fig. 10a), in the sagittal projection (Fig. 10b) the rupture zone of the implant’s own capsule is clearly visible and fibrous capsule with the formation of a gel depot in the breast tissue – external rupture of the implant.
Rice. 10.
Results of automatic volumetric scanning of the left breast of patient K., 12 years after breast surgery, external rupture of the implant.
A)
Anterior projection, coronal section.
b)
Front projection, horizontal section.
Clinical observation 7
Patient X., 42 years old, complained of asymmetry of the mammary glands. From the anamnesis it is known that endoprosthetics was performed in 2007 for cosmetic purposes, in 2020 a pregnancy occurred that ended in childbirth, the child was breastfed for up to 1.5 years. By the time of the study, lactation was completely completed. With automatic volumetric scanning depth at skin level (Fig. 11), it is possible to judge the appearance of the mammary glands with photographic accuracy.
Rice. eleven.
Results of automatic volumetric scanning of the mammary glands of patient X., 10 years after endoprosthetics.
A)
Right breast, anterior view, coronal section at a depth of 0 mm.
b)
Left breast, anterior view, coronal section at a depth of 0 mm.
On deeper sections in the right mammary gland (Fig. 12a), the endoprosthesis has an uneven, unclear contour, the contents are inhomogeneous, predominantly of increased echogenicity, shifted to the lower quadrants; in the left mammary gland (Fig. 12b) there is an endoprosthesis with an even, clear contour, the contents are homogeneous, low echogenicity, the implant is located mainly in the upper quadrants.
Rice. 12.
Results of automatic volumetric scanning of the mammary glands of patient X., 10 years after endoprosthetics.
A)
Right mammary gland, anterior projection, coronal section at a depth of 14 mm.
b)
Left mammary gland, anterior projection, coronal section at a depth of 14 mm.
In the sagittal (Fig. 13a) and horizontal (Fig. 13b) sections of the right breast we can see the details: a two-layer smooth capsule implant, the capsule itself is fragmented, the contents are heterogeneous, the gel is determined in the spaces between the intrinsic and fibrous capsules, the gel structure is cellular, the periprosthetic fibrous capsule no signs of damage.
Rice. 13.
Results of automatic volumetric scanning of the right breast of patient X., 10 years after endoprosthetics.
A)
Anterior projection, sagittal section.
b)
Front projection, horizontal section.
Clinical observation 8
Patient T., 46 years old. Condition after bilateral mastectomy: on the left for cancer, followed by long-term administration of saline to form a pocket for the implant, on the right for prophylactic purposes with simultaneous prosthetics. With automatic volumetric scanning at a scan depth of 0 mm (Fig. 14a, 14b), a skin scar is visible in the right mammary gland, the left mammary gland is smaller in size, and there is no nipple. On the right, the implant is installed under the glandular-fibrous complex, at a depth of 23.5 mm it has shallow and short folds (Fig. 14c), on the left, the implant is installed directly under the skin, the folds are also of shallow depth, but significantly longer (Fig. 14d), on both sides, the periprosthetic fibrous capsule sags over the folds (Fig. 14e, 14f).
Rice. 14.
Results of automatic volumetric scanning of the mammary glands of patient T., condition after bilateral mastectomy.
A)
Right breast, anterior view, coronal section at a depth of 0 mm.
b)
Left breast, anterior view, coronal section at a depth of 0 mm.
V)
Right mammary gland, anterior projection, coronal section at a depth of 23.5 mm.
G)
Left breast, anterior view, coronal section at a depth of 23.5 mm.
d)
Front projection, horizontal section.
e)
Front projection, horizontal section.
Conclusion
The presented observations demonstrate the possibilities of automatic volumetric scanning of the mammary glands after various types of mammoplasty, both for purely cosmetic purposes and to replenish the volume of removed tissue after surgical treatment of tumor formations. The advantages of automatic volumetric scanning over X-ray techniques are the absence of radiation exposure and the risk of rupture of the endoprosthesis due to its mechanical compression under conditions of gland fixation; over conventional ultrasound - a higher scanning frequency and, therefore, higher resolution, a full-format image of the entire gland, ease of assessment of the number and length of defects, precise topography of the “find”, high reproducibility; overcoming such disadvantages of ultrasound as subjectivity and operator dependence through standardization of installations and the process of collecting information, the possibility of post-processing and retrospective analysis.
Literature
- Fisenko E.P., Startseva O.I. Ultrasound examination of gel implants of the breast and soft tissues. 1st ed. M.: LLC "Firm STROM". 2012.
- Rozhkova N.I., Prokopenko S.P., Mazo M.L. Features of radial ductal echography of the mammary gland // Bulletin of the RAR. 2011; 4: 181–186.
- Gazhonova V.E. Ultrasonic tomosynthesis of mammary glands. M.: Prospekt, 2020.
- Jacobs O.E., Rozhkova N.I., Mazo M.L., Mikushin S.Yu. Experience of using virtual sonography of the breast // Bulletin of radiology and radiology. 2014; 1:23–32.
- Gazhonova V.E. Ultrasound examination of the mammary glands. M.: GEOTAR-Media, 2020.
- Bit-Sava E.M., Balandov S.G., Akhmedov R.M., Monogarova M.A. Radical resection of the mammary gland with plastic surgery using a musculocutaneous flap of the latissimus dorsi muscle // Bulletin of Surgery named after. I.I. Grekova. 2013:172(3):076–079.
Ultrasound machine RS85
Revolutionary changes in expert diagnostics.
Impeccable image quality, lightning-fast operating speed, a new generation of visualization technologies and quantitative analysis of ultrasound scanning data.
How does the procedure work?
Algorithm for ultrasound diagnostics of breasts with implants:
- The doctor asks the patient to undress to the waist, sit comfortably on the couch and relax.
- A conductor gel is applied to the skin of the chest, which enhances the permeability of ultrasonic waves.
- The ultrasound sensor is applied to the skin and moves across the entire area under study. The high-resolution image is visualized on the monitor screen. The diagnostician assesses the condition of the tissues of the organ and the implant, and immediately during the diagnosis voices the identified pathologies, so a preliminary diagnosis can be found out immediately.
- In some cases, the specialist may ask you to change your body position or raise your arms.
The results of the study are recorded in an ultrasound protocol, which is given to the patient immediately after completion of the diagnosis. The conclusion must be shown to a mammologist who will study the data in detail and determine an accurate diagnosis.
Indications and contraindications for diagnosis
You can sign up for an ultrasound scan of mammary glands with implants at the Hemostasis International Clinic according to your doctor’s indications or at your own request. Indications:
- painful lumps detected during palpation;
- pain, discomfort in the chest that does not go away for a long time;
- pathological discharge from the nipples with purulent, bloody, serous inclusions
- inflammation of the lymph nodes;
- elevated temperature of unknown origin;
- breast skin changes;
- pronounced asymmetry of the glands;
- planned examination of the condition of breast prostheses.
The procedure has no absolute contraindications. Diagnosis will have to be postponed for a while if there are wounds, ulcers, or rashes on the skin of the chest. Also, the procedure is not possible if the woman has a more viral infectious disease, accompanied by fever, deterioration in general health. As soon as the condition returns to normal, you can sign up for an ultrasound scan at a time convenient for you.
Ultrasound of the mammary glands
Ultrasound diagnostics of the mammary gland allows you to freely obtain images of the mammary gland in various projections, visualize the ductal and ligamentous systems of the mammary glands, and clearly distinguish tissue formations (fibroadenomas, fibrolipomas) from liquid formations (cysts, abscesses, etc.). Ultrasound is the leading method for examining the mammary glands during pregnancy and breastfeeding, with short-term planned monitoring of patients at intervals of 2-3 months, because does not have radiation exposure and is considered absolutely harmless.
It is most often performed on women under the age of 35 - at this age, a large amount of dense glandular tissue remains in the mammary glands, which is of little information for x-ray techniques - mammography.
In our Center, breast ultrasound is performed by a doctor during a consultation. To increase the reliability and information content of ultrasound (as well as other examinations of the mammary glands), doctors recommend conducting it from the 5th to the 12th day of the menstrual cycle.
Our doctors perform all medical and diagnostic procedures and work for you from Monday to Friday from 8.00 to 20.00. You can sign up for a consultation by calling: (495) 942-40-20.