Fibroblasts - what are they?
In Europe, fibroblasts have been widely used in the treatment of various diseases and anti-aging cosmetic procedures since the late 90s.
Since 2000, the technology has been developed and treatment and rejuvenation procedures are carried out in leading clinics in the USA, Britain and Switzerland using our own connective tissue cells or, scientifically speaking, fibroblasts.
The procedure cannot be called cheap - it costs from seven thousand dollars, but as the lucky ones who have experienced its miraculous effect say, the costs are worth a thousandfold.
Let's take a closer look at what this technology is and what fibroblasts are.
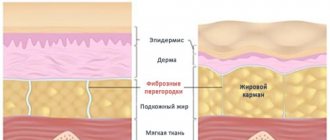
Fibroblasts in professional medicine are called cells of loose connective tissues. These cells have a special, elongated shape and look like a small spindle, from which many processes extend in different directions. Fibroblast cells are located in the middle layers of the skin, and it is from these skin cells that the epithelial framework is formed.
Briefly, we can say that the functions of fibroblasts also include the production of important biologically active substances and ensuring the full process of cellular metabolism. But in fact, fibroblasts have much more functions; we’ll talk about the beneficial qualities of skin cells in the next section of the article.
Difference from other methods
The main difference from other methods is the drug used. The patient's skin is restored using its own cells , which are cultured in the laboratory.
During the procedure, living cells are transplanted onto the skin, which dramatically accelerate the skin regeneration mechanism due to their production of growth factors and components of the dermal framework. The method not only corrects visible skin defects , but also restores it at the microtexture level.
Modern cosmetological techniques (antioxidants, hydrants, elastic stimulants, biostimulants, capillary protectors, etc.) have a certain rejuvenating effect, but the principle of action is based on correcting the effects of aging. Cell replacement therapy solves the problem of skin aging on a fundamentally different level: the process of reducing the reserves of one’s own skin cells stops. This means that the aging process in the treated area is not only stopped, but even reversed.
Regenerative therapy treats age-related skin changes when other methods only correct their consequences.
Cell functions
- Fibroblasts synthesize and release special substances into the space between cells that help the dermis maintain elasticity and firmness. Thanks to this, there is an active stimulation of the production of collagen and elastin fibers, as well as intercellular substance, which is similar to a thick gel. This gel fills the space between cells and its components are moisture-retaining hyaluronic acid and important elements such as nidogen, tinascin and proteglican;
- Fibroblasts also produce enzyme substances that can clear the intercellular space of old fibers. Thus, there is constant renewal in the layers of the dermis, and the skin remains fresh and youthful.
Cells of this type are also responsible for the synthesis of a special type of proteins that promote regenerative processes in the epithelium.
FIBROBLASTS
Fibroblasts form the extracellular matrix. They make the tissue denser and take part in wound healing. Fibroblast-like cells actively move throughout the developing embryo and give rise to a number of mesenchymal tissues. Thus, in addition to ensuring the constancy of the cell shape or its one-time stereotypical change, in addition to participating in the spreading of the cell on the substrate, the cytoskeleton of fibroblasts must also perform functions associated with active movement, cell polarization and the generation of tension. We also note that since fibroblasts are eukaryotic cells, they are capable of directed movement of substances within the cell. This expansion of the list of functions is reflected in the complication of the organization of the cytoskeleton.
The structure of the fibroblast cytoskeleton significantly depends on what phase of the cycle it is in and on what substrate it is located. Thus, the restructuring of the cytoskeleton observed during reseeding of cultured cells is comparable to that which occurs at the end of mitosis, during embryogenesis, or during wound healing. However, cultured cells are a much more convenient object for observation and experimentation. The rounded fibroblast responds to contact with an acceptable substrate by forming numerous filopodia. These thin, long processes seem to feel the space around the fibroblast. Where they touch the substrate, the process of attachment to it can begin. If contact is made with a loose particle, the filopodia often adheres to it and is pulled back together with it. As soon as the number of contacts between the cell and the substrate becomes sufficiently large, its edge appears to be covered with ripples; this process and the process of filopodia formation can replace each other. Actin at this stage is found in large quantities in the folds of the cell edge and in the thick fibers crossing the perinuclear space. As the cell continues to spread out, these fibers redistribute and form a network of polygonal-shaped cells in the interior of the cell. Over the next few hours, the polygonal actin network is rearranged into so-called tension fibers, and the cell acquires the appearance characteristic of an interphase fibroblast. The redistribution of tropomyosin occurs somewhat differently. In the early stages, when large amounts of actin are contained in cell edge folds and transnuclear fibers, virtually all tropomyosin is diffusely distributed around the nucleus. Upon completion of the formation of the polygonal network, tropomyosin is already found in it, although absent, however, at the vertices of the polygons. After network rearrangement, tropomyosin is located along the tension fibers with a period of approximately 1.5 μm. Another type of redistribution is demonstrated by α-actinin. At the earliest stages, this protein, like tropomyosin, is distributed diffusely in the center of the fibroblast. However, after about eight hours, it forms small clusters that coincide with the vertices of the actin polygons. At the locations of these clusters there are so-called focal contacts, i.e. those areas where the cell approaches the substrate at a distance of less than 15 nm. After the completion of fibroblast restructuring, α-actinin appears associated with tension fibers, located along them with the same period as tropomyosin (i.e., about 1.5 μm), but in antiphase with it, and, in addition, is concentrated in folds membranes at the edge of the cell. Several other actin-associated proteins are also found in fibroblasts. Myosin is found predominantly in tension fibers, more or less in the same places as tropomyosin; it is absent from cell microprocesses, cell edge folds, and focal contacts. One of the few proteins distributed similarly to actin is filamin. The only place where there is actin, but no filamin, is the very tips of the microprocesses. In turn, filamin is present in the space between the tension fibers; therefore, it is very likely that it can be associated in the cell not only with actin, but also with other proteins. Two actin-binding proteins, fimbrin and vinculin, are most strikingly distributed in the fully extended fibroblast. Fimbrin (mol. mass 68 kDa) was originally isolated from microvilli. A small amount of this protein is present in tension fibers, but it is mainly found at the periphery of the cell: there is a lot of it in the folds of the cell edge, microprocesses, microvilli and filopodia. Unlike fimbrin, vinculin is associated predominantly with focal contacts; in addition, some vinculin is diffusely distributed in the central part of the cell. Vinculin remains associated with the surface of the cell membrane facing the cytoplasm at the points of focal contacts even after actin has been removed from the focal contacts in one way or another. For this reason, vinculin is considered one of the proteins located at focal contacts closest to the plasma membrane. Actin in fibroblasts serves as a component of cytoskeletal structures, and each of them is characterized by its own spectrum of actin-associated proteins. At. Every serious study of the fibroblast cytoskeleton raises the same urgent question: why are different actin-associated proteins localized in different parts of the cell? For some of these proteins, restrictions in distribution may likely be due to the presence of additional binding activity: for vinculin, for example, this is the ability to bind to the membrane. Whether such an explanation will be adequate in all other cases or whether other dynamic interactions will have to be additionally taken into account will become clear only in the course of further research. The second of the main fibrillar systems of fibroblasts is the microtubule system. Microtubules converge, as if in focus, in the region of the centrioles, in the central part of the cell. Immediately after reseeding the cells, no complex network of microtubules is visible in them. However, over time, the microtubules elongate, become curved, and eventually reach the cell periphery. Microtubules are also present in the cell during mitosis; in addition, they are found in the primary cilium, a vestigial flagella-like organelle. In interphase, microtubules take part in the process of cell polarization; the cell’s ability to form folds and filopodia from only one edge and carry out directional movement depends on them. Microtubules are also needed to transport material, the extracellular matrix, from the Golgi apparatus to the outside. The third main fibrillar system in fibroblasts is formed by intermediate filaments of the vimentin type. They fill, intertwining, the central region of the cell and stretch towards its periphery. The spread of vimentin filaments throughout the cell after mitosis occurs only after the restoration of microtubules. Vimentin fibers surround the nucleus; in addition, they come into close contact with the tension fibers. Although fibroblast intermediate filaments are composed largely of vimentin, in at least one case—cardiac fibroblasts—small amounts of desmin, a protein commonly found in muscle cells, were also reliably found in the filaments. Apparently, desmin in cardiac fibroblasts copolymerizes with vimentum during the formation of intermediate filaments. Immunocytochemical methods are mainly used to study the localization of cytoskeletal proteins. The reliability of the results obtained using these methods depends both on the specificity of the antibodies used and on the accessibility of the cytoskeletal component being studied to the antibodies. The fact that immunofluorescence research methods can generally be relied upon is quite convincingly proven by experiments in which fluorescently labeled proteins were introduced into cells by microinjection. Such experiments were performed with α-actinin, vinculin, tubulin, microtubule-associated proteins, and actin. However, none of the experiments revealed any new structures different from those in which the protein used for microinjection had already been previously detected by immunofluorescence. This confirms the specificity of immunofluorescence, although, however, it does not exclude the possibility of the existence of structures that are so dense or stable that neither antibodies nor exogenous structural proteins can penetrate them. The fibroblast cytoskeleton can be examined at high resolution using an electron microscope. Some of the immunocytochemical methods have been modified for use in electron microscopy, making electron microscopic detection of individual proteins possible. Additional structural details can be revealed by using extracted cytoskeletal preparations or properly fixed whole cells. When fibroblasts are extracted with a solution of low osmotic pressure, many fibrillar structures are preserved and can be identified by the immunoferritin method. Actin filaments are visible associated with each other, as well as with microtubules and intermediate filaments. In addition to these three main types of fibrillar structures, such cytoskeletal preparations reveal numerous heterogeneous filaments that cross-link the filaments of the three main systems with each other. Under milder conditions, when cells are extracted in the presence of sucrose, which protects them, an even more complex network can be revealed. In such a network, the filaments are arranged so densely and sometimes have such a small diameter that it is not possible to distinguish them on ordinary thin sections of the cell. Finally, a very complex picture, including the finest, variable microtrabeculae associated with both the filaments of the main tiggi and intracellular organelles, is observed when thick sections of intact cells or directly whole cells grown on substrates for electron microscopy are examined using high-voltage electrons. The increase in the complexity of fibrillar structures as a result of measures to protect the cytoskeleton during drug preparation possibly reflects differences in the duration of residence of different proteins in the cytoskeleton. In fact, those proteins that are included in the cytoskeleton for a short time (but quite often) will be detected in the preparation only using methods that ensure stabilization of their connection with the cytoskeleton, whereas in the case of significant extraction, predominantly those proteins for which exchange with the soluble phase of the cell occurs rarely.
Related materials:
Muscles
Epithelial and endothelial cells
Transformed cells
Protista
Application of fibroblasts in cosmetology
Fibroblasts have found their use in procedures aimed at rejuvenating and slowing down the natural aging processes of the dermis. It is known that the aging process of the dermis enters an active phase from the age of 25-30 years and in this process changes occur primarily at the cellular level.
Aging begins with a decline in the activity of fibroblast cells: they stop synthesizing the required amount of collagen and elastin fibers, and the level of hyaluronic acid drops to a critical point.
Of course, these hidden processes are reflected in the condition of the dermis: it becomes thin, dries out, and takes on a pale or grayish tint. The epithelium also loses its firmness and elasticity, as a result of which wrinkled networks appear on the skin, which gradually grow and acquire depth, turning into age-related folds. And the older a person is, the faster the pace of development of these negative processes. Age-related changes appear most quickly in the neck, face, décolleté and arms.
Thanks to cellular technologies, the aging process can be, if not stopped, then significantly reduced in intensity. Stimulation of fibroblast cells fights aging from the inside and that is why procedures using cells of this type are so effective.
SPRS will restore youth to your skin!
How can SPRS restore youthful skin? Our skin is made fresh, youthful and elastic by so-called fibroblasts - special cells of the dermis. As we grow and age, there are fewer and fewer of these cells in the body: fibroblasts lose activity, do not produce collagen and elastin fibers that form the skin frame as before, and also produce insufficient hyaluronic acid, which keeps the dermis moisturized. Because of this, it loses thickness and elasticity, gradually starting to become covered with wrinkles.
SPRS therapy is a carefully selected individual set of procedures for the diagnosis, treatment and restoration of skin, the essence of which is the use of only a person’s own fibroblasts (fibroblasts). This technique was developed by specialists from HSCI, a leader in the application of cellular technologies in Russia. The Institute is the first organization to which RosZdravnadzor issued permission to introduce this technology for restoring youth.
Fibroblast injections: the essence of the procedure
The essence of the procedure is quite simple: young connective tissue cells are transplanted into the dermis. Moreover, the method uses one’s own cells, which allows not only to avoid a rejection reaction, but also to maximally accelerate the processes of renewal and regeneration of the skin. The result of the procedure will be a noticeable improvement in complexion, reduction of wrinkles of all types (from facial to deep), deep hydration of the dermis, healing of scars and post-acne.
The main advantage of the technology is that fibroblast injections are highly effective and retain their beneficial qualities for a long time.
The results from the procedure will last for at least six months!
And all this time, collagen, elastin and hyaluron will be actively produced in the dermis and in the required quantity.
The cells for injection are obtained from the patient's dermis, a small piece of which is usually taken from the navel area or from the area behind the ears. It is in these areas of the human body that the skin is least susceptible to the negative effects of environmental factors. This piece is carefully examined and processed in the laboratory, stimulating the growth of young fibroblast cells. On average, cell cultivation takes a month to a month and a half, after which the grown cells are injected into the clinic patient’s area in need of correction.
A pronounced result can be expected after the first autotransplantation session, but to achieve a significant effect, cosmetologists advise taking a two-week course. Six months after the procedure, the depth of age-related changes in the area around the eyes is reduced to almost ninety percent, around the mouth - to fifty-five percent, on the neck and chest area - to ninety-five percent.
The process of introducing cells into a problem area
The schedule for introducing cells into the problem area of the skin depends on:
- the time required to obtain a cell culture;
- therapeutic dose of a living cell drug;
- individual characteristics of the patient's skin;
- from choosing the optimal treatment regimen recommended by the doctor.
Cell injection area
The area into which fibroblasts are injected intradermally is determined jointly by the doctor and the patient. As a rule, problem areas of the face are selected - around the eyes, nasolabial folds. The cell preparation can also be injected into the neck and décolleté area.
Of particular interest is the use of autologous fibroblasts for rejuvenating the skin of the dorsum of the hands. Autologous fibroblasts can be injected into the area of scars, stretch marks, and wrinkles.
Depending on the depth and severity of skin changes, a papular or tunnel method of administering the cell preparation is used.
Live cell injection procedure
- the procedure is performed under local anesthesia. The doctor injects a drug enriched with fibroblasts into the patient’s problem area with a thin needle;
- depending on the problem, several sessions may be required;
- the procedure is performed under local anesthesia. Using a thin needle, the doctor injects a drug enriched with fibroblasts into the patient’s problem area;
- After the procedure, slight redness and swelling may occur, which will disappear within 2 - 3 hours.
When active fibroblasts are introduced into the skin, the natural processes of restoration of the dermis are launched. The effect of the procedure begins to appear 6-8 hours after the session and increases over time. 8 - 9 months after the course of treatment, a persistent positive result is observed.