Placenta Laennec is used worldwide to treat more than 80 different diseases
- allergies and asthma,
- prostate adenoma,
- hair loss,
- symptoms of menopause, especially in women who refuse HRT,
- male menopause,
- metastases,
- metabolic disorders, enuresis,
- high and low blood pressure,
- ulcers and stomach cancer,
- effectively heals wounds, fights keloid scars,
- chronic fatigue,
- painful menstruation,
- infertility,
- ovarian dysfunction,
- skin diseases,
- constipation,
- neuralgia, migraines and neuroses,
- anemia,
- osteochondrosis, etc.
At Platinental, we achieve amazing aesthetic results using Laennec IVs for preoperative patient preparation and rapid postoperative rehabilitation.
Why has Laennec gained such recognition in world medicine? Not only for its rich composition.
We all know that when taking medications, other organs suffer in one way or another. And we “by default” accept these rules of the game.
Being a new generation product, Laennec is radically different from drugs! This is a very high quality drug with intelligence. “Laennec” itself finds weak links in the body and takes measures to eliminate them.
Therefore, it shows
excellent results in treating various diseases without causing any harm!
Features of drug production
The drug Laennec was created by the Japanese doctor Hieda Kentaro. But if not for the research of Russian ophthalmologist Vladimir Petrovich Filatov, this medicine might never have appeared. It was his experiments that became the basis of tissue therapy.
Filatov began developing a new treatment in 1930. He came to the conclusion that the cells of some tissues separated from the body are capable of producing biogenic stimulants. If these tissues are implanted into the patient’s body, they can have a powerful healing effect.
For example, 4 mm of cadaveric cornea transplanted into the patient's body accelerated regeneration in the patient's affected cornea. By transplanting pieces of skin, spleen or cartilage, Filatov was able to heal patients with polyarthritis, gastric ulcers, burns, bronchial asthma and many other diseases.
In addition, he discovered that the affected cells in the body are revived under the influence of growth factors contained in the placenta. This discovery saved thousands of lives during the Great Patriotic War.
In 1945, Filatov’s work came to the future creator of the drug Laennec. The Japanese government was tasked with creating a product that could quickly restore the health of the population. This was especially true after the atomic bombing.
For a long time, it was not possible to properly clean the placenta from blood. Thanks to the electronic computer breakthrough, it was possible to create the necessary processing technology. The resulting natural substrate met all the requirements of the highest medical tests for purity.
Therapy with Laennec is completely safe. This was achieved not only thanks to the high degree of purification of the material, but also to the strict rules that the female donor must adhere to:
- Women who have visited countries where cases of infectious diseases in cattle have been reported are prohibited from participating in the program.
- During the conclusion of the initial contract, the female donor fills out a questionnaire in which she indicates all the information about trips abroad and all the diseases she has suffered.
- It is prohibited to leave Japan before the placenta is collected.
- A candidate donor must be tested for the presence of prion proteins in the blood, all types of hepatitis virus, HIV, papillomavirus, syphilis, etc.
- The donor can terminate the contract at any time if he wishes.
Nonsense, you say? Rave?
Are you smiling skeptically because there is no cure for all diseases?
Let's look at the facts.
Since 1956, Laennec has been successfully used within the framework of the State Program for the Improvement of the Nation of Japan. During this time, the average life expectancy of a Japanese person has increased from 63 to 82 years and over the past 35 years has consistently overtaken Sweden, which traditionally has the highest standard of living.
Have you noticed that the higher you climb the mountain of success, the fewer competitors there are around? On this difficult path, many competitors “burn out”: they develop neuroses, become drunkards, and exhaust their strength with artificial stimulants.
Only the healthiest and smartest are at the top of the world. Those who take full advantage of the achievements of modern medicine for their benefit.
The secret of Laennec's effectiveness is in its production
The production of Laennec is based on a whole philosophy.
Thus, the placenta for the drug is taken only after successful natural
childbirth But this is not the only condition. It is important that the pregnancy is desired by both parents, and that the born child is full-term and healthy.
To ensure safety, the placenta undergoes many tests at different stages of its processing. The finished placental extract is additionally subjected to sterilization.
Donors who consented to the use of the placenta are healthy and are monitored by a doctor throughout the pregnancy. Subsequently, they are under close medical supervision for several years and are regularly tested for infections.
Placental IVs Laennec
Different methods are used to administer Laennec:
- intravenous;
- intramuscular;
- injections using mesotherapy at acupuncture points.
Droppers are considered the most effective.
Laennec droppers affect problem areas of the body at the cellular level.
Placenta-based drugs improve heart function and increase calcium absorption (which is especially important during the period after menopause), stimulate insulin production and metabolic processes, and prevent the deposition of salts and cholesterol on the walls of blood vessels. Laennec restores vitality, performance, psychological stability and significantly improves the quality of life.
In fact, Laennec droppers awaken the body's reserves, stimulate the active synthesis of enzymes and hormones, thereby reducing your biological age.
Release form and composition
The medicine is available in a single form - as an injection solution. It is a transparent liquid of a yellowish tint, which can be either light yellow or brown, with a specific odor. The product is bottled into dark glass ampoules (2 ml each), and then packaged in cardboard packs of 10 pieces. You can purchase goods at a pharmacy only with a doctor’s prescription. The medication is not intended for independent use. An IV is required to administer Laennec. The procedure should be performed by an experienced medical professional.
As noted above, the main active ingredient of the drug Laennec (Japan) was the hydrolyzate of the human placenta. One ampoule contains 112 mg. The medicine also contains additional ingredients . Among them:
- hydrochloric acid to adjust pH levels;
- water for injections;
- hyaluronic acid;
- more than 40 minerals (phosphorus, zinc, sulfur and others);
- various vitamins;
- 18 amino acids;
- about 100 enzymes;
- interleukin complex;
- 11 cellular growth factors.
Today it will not be possible to find any other drug with a similar composition on sale. Therefore, we can say with confidence that the product has no analogues.
What is needed to start treatment
Placental therapy is carried out after reaching 18 years of age as prescribed by a doctor.
The examination includes:
- general blood analysis,
- tests for infections (syphilis, HIV, hepatitis B, C),
- hormones (GH, ACTH, thyroid hormones, sex hormones),
- Ultrasound of the abdominal cavity, kidneys, thyroid gland, pelvic organs,
- immune status,
- detailed biochemical blood test.
Research by the World Health Organization specifically recommends regularly, 2 times a year, taking the Laennec course between 35 and 45 years. At this age, the body's biological activity reaches its peak. The use of placenta during this period prolongs productive maturity and delays the onset of old age by years.
Detailed description of the drug Laennec
The drug "Laennec" (Japan Bioproducts Industry o., Ltd., Japan) is a specially purified hydrolyzate of human placenta. Laennec has hepatoprotective properties, its effectiveness in treating skin lesions (psoriasis, acne, herpes, etc.) has been proven; in addition, the drug is actively used in cosmetology. Such a wide range of its applications is due to its composition. Laennec, being an extract of the human placenta, contains egg biologically active substances. These include growth factors: HGF, NGF, EGF, - FGF, G-CSF, M-F, IGF(-l), TGF-B1, PDGF-BB, VEGF, TNF-a, interleukins 1-6, 8, 10, 12; IFN-7, leptin, DHEA. In addition, the drug contains 18 amino acids, the content of which is 35.7 mg/ml, peptides, nucleic acid bases, sugars and other compounds, and therefore pharmacokinetic studies of the drug are not possible.
According to the results of preclinical and clinical studies conducted in Japan, the drug has the following pharmacological properties:
- Stimulates liver regeneration (proliferation of hepatocytes);
- Lipotropic ability (reduction of total lipid and cholesterol content in the liver);
- Improves tissue respiration (increased activity of succinic acid dehydrogenase in the liver of rats);
- Action that accelerates the absorption of connective tissue (suppresses the development of fibrosis).
Numerous clinical studies of the drug "Laennec" conducted in Japan, used for almost the entire range of liver pathologies, confirmed the effectiveness of the drug in patients with acute and chronic viral hepatitis, cirrhosis of the liver and fatty liver of alcoholic and non-alcoholic origin, cholangitis. All observations noted: general improvements, positive dynamics of biochemical parameters, and a decrease in the clinical manifestations of the disease. Side effects (pain at the injection site and isolated cases of redness, itching and numbness at the injection site, gynecomastia) were reported in 3.7%.
The purpose of this study is to evaluate the effectiveness, tolerability, and safety of the drug “Laennec” (injection solution 4 ml for intravenous drip in 500 ml of 5% glucose solution according to the scheme of 1 injection per day for 14 days) in patients with alcoholic and non-alcoholic steatohepatitis.
The objectives of the study were:
- Evaluation of the therapeutic effectiveness of the drug "Laennec".
- Assessment of the tolerability and safety of the use of the drug "Laennec" (solution for injection 4 ml) with intravenous drip administration in 500 ml of 5% glucose solution according to the scheme of 1 injection per day for 14 days in patients with alcoholic and non-alcoholic steatohepatitis.
Inclusion criteria:
- Patients with steatohepatitis (alcoholic and non-alcoholic origin)
- A biochemical blood test determined an increase in biochemical markers of liver diseases characterizing steatohepatitis (AST and ALT, and/or gamma-GT, and/or alkaline phosphatase, and/or bilirubin) by 2 or more times.
- An ultrasound examination revealed signs of steatohepatitis.
- Men and women aged 18 to 60 years.
- Signed informed consent to participate in the study.
Exclusion criteria:
- Age less than 18 years, more than 60 years.
- Pregnancy (in particular planned), lactation);
- >Ongoing alcohol abuse;
- The need to take corticosteroid drugs, antiviral drugs and immunosuppressants during the study;
- Hypersensitivity to Laennec;
- Malignant neoplasms of any location;
- Inability to start treatment within two days after the initial study;
- Patient participation in any other study.
The presence of severe concomitant psychoneurological, cardiovascular, pulmonary, renal and other somatic diseases;
Material and research methods
30 patients, 21 men and 9 women, were accepted for the study. The average age was 47.7±3.6 years. Depending on the etiological factors, patients were distributed as follows (Table 1).
Table 1
Distribution of patients depending on the etiology of steatohepatitis
| Etiology of steatohepatitis | Number of patients |
| Obesity | 8 |
| Alcohol abuse | 14 |
| Viral hepatitis C | 2 |
| Mixed etiology | 6 |
As can be seen from the table, obesity, as a cause of steatohepatitis, was identified in 8 patients (body mass index was above 30). Alcohol abuse, identified by a special questionnaire to establish hidden addiction to alcohol (2 or more points scored), was found in 14 patients with a normal body mass index. In 2 patients, the presence of signs of steatohepatitis, according to ultrasound, was associated with the presence of viral hepatitis C. As is known, fatty hepatosis is often detected in this pathology. Some patients had a mixed etiology - 6 patients. In this subgroup, 5 patients had a body mass index above 30, and they scored twice as high when filling out a questionnaire to identify hidden alcoholism, and 1 obese patient suffered from viral hepatitis C.
The duration of exposure to the etiological factor (obesity, alcohol abuse, viral hepatitis C) averaged 9.9+2.5 years. The duration of the disease, according to the anamnesis, was 2 times less and amounted to 4.2 ± 1.6 years.
Treatment scheme
For clinical trials, the drug "Laennec" was used in dosage form: solution for injection in ampoules of 2 ml. The daily dose of Laennec in this study was 4 ml (2 ampoules). The dose was administered once, entirely, in 500 ml of a 5% glucose solution (2 ampoules of Laennec were diluted in 500 ml of a 5% glucose solution ex tempore, immediately before administration). The injection was carried out intravenously into the cubital vein at a rate of 90-120 drops per minute. Before administration, a three-fold biological test was carried out. The drug was administered daily for 14 days.
Performance evaluation criteria
The effectiveness assessment was carried out taking into account a number of indicators.
- Subjective indicators: complaints, self-esteem by the subjects.
- Objective clinical indicators. General condition, skin, sclera. The presence of edema, ascites, signs of portal hypertension, hemorrhoids. Palpation of peripheral lymph nodes, determination of body temperature, respiratory rate, heart rate, blood pressure, body mass index (BMI = MT/F, where MT is weight, kg; P is height, m), the degree of development of subcutaneous fat. Palpation of the abdomen, edges of the liver, gall bladder area, spleen. Determination of the size of the liver, characteristics of the edge of the liver and the degree of its protrusion from under the edge of the costal arch.
- Laboratory parameters (AST and ALT, gamma-GT, alkaline phosphatase, bilirubin, cholesterol).
- Signs of steatohepatitis observed during ultrasound examination.
- Absence of pathological symptoms at the final stage of the study and normalization of the clinical picture.
Evaluation of the effectiveness of treatment included subjective, objective and laboratory research methods and was carried out by comparing data from primary, intermediate and final clinical, laboratory and instrumental examinations.
At the end of the trial, the researcher made an overall assessment of the clinical effectiveness of the drug on a four-point scale:
- Poor effectiveness, absence or negative dynamics of clinical and/or biochemical parameters;
- Satisfactory effectiveness, slight improvement in clinical and/or biochemical parameters;
- Good, treatment significantly improves clinical and/or biochemical parameters;
- Excellent, treatment leads to normalization of clinical and biochemical parameters.
Statistical processing methods
Statistical processing of data from clinical, laboratory and instrumental studies was carried out on an IBM computer in a semi-automatic mode using the standard MS Excel software package using variation statistics methods taking into account the Student's t-test. Statistical analysis of individual dynamics for each patient was performed using the difference method.
Research results and discussion
The nature of complaints and their dynamics under the influence of treatment (by the 14th day) are presented in Table 2.
table 2
Complaints of patients and their dynamics at the end of treatment.
| Complaints | Before treatment (number of patients) | After treatment (number of patients) |
| Pain/discomfort in the right hypochondrium | 17 | 2 |
| Weakness | 17 | 1 |
| Fatigue | 11 | 4 |
| Nausea | 4 | 0 |
| Heartburn | 4 | 0 |
| Belching | 4 | 0 |
| Decreased appetite | 3 | 0 |
| Frequent stools | 2 | 1 |
| Flatulence | 5 | 0 |
| Pain along the colon | 1 | 1 |
As can be seen from the presented data, symptoms of the gastrointestinal tract by the end of treatment disappeared in almost all patients, weakness persisted in 1 patient, fatigue persisted in 4 out of 11 patients. Objective clinical indicators (general condition, skin, sclera coloration, heart rate, respiratory rate, blood pressure) were initially unchanged. In all patients, this picture persisted after treatment. No edema, ascites, or signs of portal hypertension were observed.
The dynamics of laboratory parameters—markers of stedropatitis—are presented in Table 3.
Table 3
Dynamics of biochemical parameters before and after treatment with Laennec
| Index (norm) | Before treatment | After treatment |
| ALaT (7-53 units) | 99,8+8,7 | 51,6+10,0* |
| AsAt (11-37 units)* | 69,7+7,9 | 38,3±6,6* |
| Gamma GT (11-50 units) | 140,5±10,3 | 71,9±6,4* |
| ALP (100 – 290 units) | 154,3±9,3 | 145,0+8,2 |
| Bilirubin (0-22 mmol/l) | 15,3+0,9 | 14.3±0.b |
| Cholesterol | 5,5+1,0 | 5,3+0,6 |
As can be seen from table. 3, a significant decrease in transaminases and gammaglutamtranspeptidase was observed. Phosphatase and bilirubin levels were within normal limits before and after treatment. There was a tendency towards normalization of cholesterol, but the data are not reliable.
The drug was well tolerated in all patients; the effectiveness results are presented in Table 4.
Table 4
Evaluation of the effectiveness of treatment with the drug "Laennec"
| Efficiency | Efficiency |
| Badly | 2 (6,9%) |
| Satisfactorily | 3 (10,3%) |
| Fine | 17 (58,6%) |
| Great | 7 (24,2%) |
A study of the effectiveness and safety of the drug "Laennec" showed that the drug is effective in the treatment of steatohepatitis of alcoholic, non-alcoholic (associated with obesity) and mixed etiology. There was a normalization of physical well-being, improved biochemical parameters, and some patients showed positive dynamics in the ultrasonographic picture. The effect of the drug in this group of patients was rated as good or excellent in 82.8% of cases. On the other hand, in patients with ultrasound signs of hepatosis and the presence of hepatitis C virus, the effect of the drug was assessed as poor (2 cases) and satisfactory (1 case), while in 1 case there was no positive clinical dynamics, negative biochemical dynamics were observed (increase in transaminases) in 2 cases.
The results of the study indicate that the drug "Laennec" can be recommended for use as a powerful hepatoprotector for the treatment of steatohepatitis of alcoholic, metabolic and mixed etiology. The drug was well tolerated and had a positive result by the 14th day of treatment, however, complete normalization of biochemical parameters did not occur during this period, which, apparently, may require an increase in the duration of treatment in some cases. The drug was well tolerated in all cases, no side effects were observed.
Patients with viral hepatitis C (3 patients) represent a group that is qualitatively different from patients with steatohepatitis of alcoholic, metabolic and mixed etiology. The inclusion of patients with viral hepatitis C in the study was somewhat random - according to the protocol, treatment should be started no later than 2 days after the examination, and the results of the virological study were obtained after 7-10 days. The study was not aimed at assessing the effectiveness of Laennec in patients with chronic viral hepatitis, but the established precedent prompted us to study the literature data. As it turns out, the drug Laennec has been used in Japan since the 70s. last century in patients with chronic hepatitis and cirrhosis, including viral etiology with good effect. Moreover, with the introduction of virological diagnostics into widespread practice, reports appeared about the normalization of biochemical parameters and the disappearance of hepatitis C RNA after a 3-6-month course of monotherapy with Lennec. In this case, the drug administration regimen differed from that used by us and was 2 ml 3 times a week (1, 2). Thus, studying the possibility of using the drug “Laennec” in patients suffering from viral hepatitis C (in case of ineffectiveness, intolerance, contraindications to antiviral therapy, etc.) seems interesting.
Conclusion
The drug "Laennec" is effective in the treatment of patients with alcoholic and non-alcoholic steatohepatitis. The average duration of treatment with daily intravenous administration of 4 ml (2 ampoules) is 2 weeks, but in some patients it can be increased if necessary. The drug is well tolerated, no side effects were observed.
Contraindications
- pregnancy and breastfeeding period,
- childhood,
- hypersensitivity to the drug,
- allergies to medications.
Review:
“Lannek, or as it has become popular among patients, “placental drip”, was created specifically for residents of a big city.
Our rhythm of life, harmful factors (air pollution, alcohol, smoking) - all this creates preconditions in the body for the emergence of tumor cells, the occurrence of chronic fatigue syndrome, deterioration in skin quality, and so on.
I do droppers with Laennec in a course of 5 procedures 2 times a year as a preventive measure and to strengthen the immune system in spring and autumn. Do not forget that rejuvenation is a systemic process. And when performing plastic surgery to lift the face or body, do not forget that the internal resources of our body also need to be recharged.”
Andrey Iskornev, plastic surgeon, president of The Platinental Aesthetic Lounge
Contraindications and side effects
The use of the drug Laennec (Laennek, Linek) in cosmetology is prohibited:
- children under 18 years of age;
- during pregnancy and lactation;
- if you are allergic or sensitive to one of the components of the drug;
- patients with cancer.
In rare cases, side effects of the drug may occur . At the time of the study, side effects were found in no more than 4% of patients. After administration of the drug, compactions, redness of the skin, soreness, itching, breast enlargement in men, and hematomas may be observed, which disappear over time.
It is important to know ! To prevent side effects, an allergy test to the components of the drug is performed to avoid the occurrence of anaphylactic shock.
Laennec effect in detoxification
"Laennec" today has earned itself the reputation of an unsurpassed hepatoprotector
– means of treating and strengthening the liver. It removes waste and toxins from the body and mobilizes the body's defenses.
The drug is used to treat various hepatitis, cirrhosis and alcoholic liver damage, normalize fat metabolism in the liver in case of excess weight.
Indications and contraindications for use
The unique composition of Laennec and the peculiarities of its effect on the body explain its wide range of applications.
Indications for placental therapy are:
- skin diseases (psoriasis, dermatitis, herpes);
- allergic reactions;
- menopausal syndrome;
- liver pathologies (hepatitis, cirrhosis, etc.);
- infertility, preparation for IVF;
- sleep disorders, nervous tension, stress, depression;
- decreased immunity.
In cosmetology, “Laennec” is indicated in the following cases:
- wrinkles;
- dryness, sagging skin;
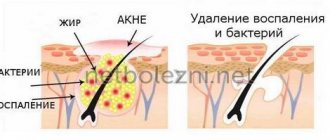
- acne;
- dark spots;
- hair loss.
Despite the obvious benefits of the drug, there are a number of contraindications to its use:
- age up to 16 years;
- pregnancy and breastfeeding;
- individual intolerance to components.
Numerous clinical studies of the drug have shown that in extremely rare cases the following side effects may be observed during therapy:
- allergic reactions;
- pain, short-term numbness at the injection site.
Patients who have completed a course of injections or droppers “Laennec” note that not only the main ailment goes away, but also an improvement in the functioning of all body systems is felt, from improving general well-being to normalizing the condition of the skin and hair.
Compatibility with alcohol
To avoid toxic effects, experts recommend completely abstaining from alcohol during treatment with Laennec.
Can I take it during pregnancy?
Studies have proven that the substances included in the drug are able to penetrate the placenta and have a negative effect on the development of the fetus. Gynecologists recommend stopping taking the drug during pregnancy and breastfeeding.
Rejuvenation "Laennec"
Rejuvenation is a complex process. It’s not just our toned face that speaks of youth. Mobility, activity, flexibility, strength, reaction speed - the body's internal resources also need to be recharged.
Do you live in the city? Then you are familiar with insomnia, stress, gas pollution, chronic lack of sleep, eating on the go, headaches, allergies... In combination with alcohol and smoking, these factors contribute to early aging. They literally kill the immune system, dry out the skin, lead to chronic fatigue, and wear out the body.
The placenta components in Laennec are a rare case when one medicine helps in the treatment of big city syndrome. They activate and nourish the body’s own forces, returning it to a state of youth.
“As a result of such exposure, the cells of all systems and tissues are activated, and rejuvenation occurs,” writes Professor Yoshida Kentaro in the book “The Power of the Placenta.” – The placenta strives to bring all body functions as close as possible to their state at a young age. This is the secret of the magic potion of youth and longevity.”
Even one Laenek procedure triggers the renewal of body cells. Sick, defective, worn out, old and damaged are replaced. It is also important that Laennec does not allow the division of such defective cells. Thus, Laennec not only rejuvenates, it effectively prevents the appearance of tumors and slows down the growth of cancerous formations.
Analogues and prices
The only analogue of the drug Laennec can be considered another placental Japanese product called Melsmon. It also consists of hydrolyzed human placenta. The composition of the drug includes:
- 16 amino acids;
- mucopolysaccharides;
- low molecular weight peptides;
- organic and nucleic acids;
- minerals involved in the formation of hemoglobin and the construction of the skeleton.
The indications and contraindications for the drugs are identical. Experts say that Laennec is more suitable for instantly eliminating intoxication. If you need to transform your skin, hair and nails, it is better to use Melsmon.
The cost of a package of Laennec, consisting of 10 ampoules, is 12,500 rubles. Melsmon will cost more: for 10 ampoules you will have to pay 20,500 rubles. Medicines are often counterfeited, so you need to buy them only from trusted pharmacies.
"Laennec" in cosmetology
"Laennec" is effective against psoriasis, atopic dermatitis, herpes, acne, and age-related skin pigmentation.
For intensive hydration, another unique property of the placenta is used - it retains water in the skin in a volume six times greater than the volume of the injected drug.
But these are not all the advantages of the new drug. It turns out that the color of our skin is largely determined by the condition of the liver. In this case, the unpleasant shade cannot be affected by cosmetic procedures. Only Laennec, acting simultaneously on the liver and skin, is guaranteed to return our face to the color of spring sakura.
Review:
“Professional makeup, flights, lack of sleep. Without this, there is no life as a TV presenter. But the viewer doesn't need to know about this. Then effective products with a WOW effect come to the rescue. Laennec is one of them.
Mesotherapy with Laennec for the face is an instant recovery, deep hydration and long-lasting effect.
I am delighted!"
Tatyana Arno, TV presenter.
Instructions for Laennec
For atopic dermatitis and recurrent chronic herpes, the drug is administered intravenously by drip: 10 ml (five ampoules) are dissolved in 260-500 ml of saline or 5% dextrose solution. Injections are made at intervals of 2 days. The course of therapy includes 10 such administrations.
For chronic liver damage, it is recommended to administer the drug intramuscularly, 2 ml per day. The frequency of injections, if necessary, can be increased to 3 times a day. The drug is also allowed to be administered intravenously as described above. The course of therapy is up to 3 weeks.