Proteoglycans are new possibilities for the treatment of alopecia. Congress of the Russian Society for Hair Research
The relevance of the problem of alopecia is associated not only with the increase in its prevalence, the lack of a clear picture of pathogenesis, but also with the insufficient effectiveness of treatment. One of the most promising modern trends in trichology - replacement therapy with proteoglycans - was the subject of a speech by the professor of the Department of Dermatovenereology of the First St. Petersburg State Medical University. acad. I.P. Pavlova, Doctor of Medical Sciences Elena Alexandrovna ARAVIYSKAYA at the session “New in Therapy”, organized as part of the congress of the Russian Society for Hair Research (St. Petersburg, June 22, 2020). In her speech, the speaker, using the example of the drug Nucrrin, demonstrated the role of proteoglycans in normalizing the hair growth cycle.
E.A. Arabian
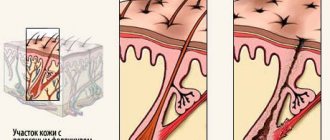
According to Professor E.A. Arabian, in order to understand what proteoglycans are, you need to remember the structural features of the dermis. The dermis is represented by two layers, vaguely delimited from each other: papillary and reticular. The papillary layer is formed by loose connective tissue, the reticular layer is formed by dense, unformed fibrous tissue. The dermis consists of various cellular elements, intercellular matrix, and fibers.
The intercellular matrix (ground substance) has an amorphous structure and has a low electron density. It is composed of proteins (collagen and elastin), glycosaminoglycans (GAGs), glycoproteins and proteoglycans1.
In the intercellular matrix, collagen fibers are assembled from collagen. Collagen is a complex of 11 proteins. It is the basis of connective tissue and provides its strength as well as elasticity. In adults, type 1 collagen predominates, in children - type 3.
Elastin is the main structural protein of elastic fibers (2–3% of skin dry weight). It is synthesized by fibroblasts and endothelial cells. Elastic fibers, which are formed in the intercellular space from elastin, bind to collagen fibers and hyaluronic acid, creating a three-dimensional structure of the skin.
Hyaluronic acid is a non-sulfated GAG that is synthesized by the enzyme complex of plasma membranes. The GAG family also includes sulfated GAGs, which are synthesized in the Golgi complex of fibroblasts. The biological role of GAGs is to interact with collagen molecules, retain water, etc.1
All glycoproteins are united under one name “glycoconjugates”2. Glycoconjugates are proteins containing a carbohydrate component covalently attached to a polypeptide backbone. The carbohydrate content of these proteins can vary from 1 to 85%.
The biochemical difference between glycoproteins and proteoglycans lies in the proportion of the carbohydrate component: for glycoproteins it is small, for proteoglycans it is large. In glycoproteins, the share of carbohydrates averages 15–20%. They do not contain uronic acids and their carbohydrate chains are short. The range of functions of glycoproteins is quite wide - from structural, protective, receptor, hormonal to enzymatic and transport.
Carbohydrates account for 80–85% of proteoglycans. Proteoglycans are distinguished by the presence of uronic acids (in particular, glucuronic acid, glucosamine, galactosamine) and long carbohydrate chains. The connection with the protein occurs through serine and asparagine2.
According to classical concepts, the functions of proteoglycans are filling the intercellular space, retaining water, forming collagen fibers, and ensuring communication between the cell surface and the components of the intercellular matrix.
It has now been established that there are more than a thousand different types of proteoglycans in human connective tissue. Proteoglycans bind to a number of other proteins, including growth factors. This leads to the localization of growth factors at specific tissue sites and their protection from degradation by extracellular proteases3, 4.
With age, the composition of proteoglycans changes. As a result, the free water content in the dermis increases.
Next, Professor E.A. Arabian briefly described the structure of hair. The hair consists of a shaft protruding above the skin and a root located in the hair follicle, immersed inside the dermis and subcutaneous fatty tissue. The hair follicle is surrounded by a connective tissue hair follicle.
According to research results, the hair follicle is a reservoir of epidermal stem cells (bulge)1, 4. The follicle is subject to cyclic changes. Reorganization of the hair follicle occurs as a result of a series of inductive interactions between mesenchymal and epithelial cells. Mesenchymal cells in the hair papillae initiate hair growth.
An important role in inducing different phases of the life of the hair follicle is played by factors such as insulin-like growth factor 1 (IGF-1), fibroblast growth factor 7 (EGF-7), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF).
Growth factors are divided into anagen-promoting and apoptosis-promoting5. Among anagen promoters, special attention is paid to Wnt. This name was formed by combining the names of two genes – Wg + Int. The Wg (wingless) gene was discovered in Drosophila; a mutation in the gene suppressed the development of wings. The Int gene is a homologous gene in vertebrates and is associated with the development of cancerous tumors.
Wnt is one of the most important molecular signaling pathways that regulates embryonic development and cell differentiation6, 7. Activation of the Wnt signaling pathway is essential for hair follicle development.
A number of studies have shown the role of the Wnt-7 ligand, one of the components of the signaling pathway, in the formation of new follicles. Thus, experimental studies in mice revealed overexpression of Wnt-7. As a result, the area on which new hair follicles were formed doubled. When Wnt signaling is disrupted, the hair follicle is delayed in the telogen phase and, as a consequence, hair growth stops7–9. Therefore, activators of Wnt signaling can be considered as potential agents against alopecia.
The role of proteoglycans in regulating hair growth is diverse. Proteoglycans form a specific microenvironment in the hair follicle with a reservoir of growth factors that help maintain homeostasis and control hair follicle regeneration10.
Specific proteoglycans such as versican, decorin, and syndecan are involved in regulating the development cycle of the hair follicle. Versican is able to increase or suppress the biological activity of secreted growth factors. Syndecan regulates the Wnt signaling pathway. Decorin is a series of hair growth factors3, 4.
Versican is involved in the anagen phase. It has been established that the peak content of versican occurs precisely in this phase. During catagen and telogen, its level decreases significantly11. Recent research suggests that selective activation of the versican promoter during hair follicle development may stimulate the hair follicle to enter the anagen phase. Even one chain of versican can stimulate the anagen phase12.
Decorin regulates transforming growth factor β (TGF-β), epidermal growth factor (EGF), IGF-1 HGF and is a signaling molecule for all known participants in the hair follicle life cycle, and also acts as an anagen phase inducer4. Decorin was found to be highly expressed in the bulge region. With age, its expression in the bulge decreases, and at the same time the level of KRT+ stem cells decreases. Therefore, decorin is an important regulator of stem cell activity13.
The syndecan family, particularly syndecan 1, also regulates anagen phase activity, and its activity decreases as the follicle involutes4. Proteoglycan levels may decrease not only with age, but also under the influence of exposome factors such as stress14.
Dysregulation of hair growth may be associated with prolongation of the telogen phase, shortening of the anagen phase, the presence of autoimmune inflammation, etc. Therefore, to treat alopecia, you need universal remedies that allow you to normalize the natural hair growth cycle.
Proteoglycan replacement therapy is a promising and modern approach to solving the problem of hair loss.
Nucrine is a new generation universal remedy for replacement therapy of various forms of alopecia, helping to normalize the natural hair growth cycle. Thanks to a course of taking the Nucrrin product, hair follicles in the telogen phase receive the structural components necessary to initiate the growth phase. As a result, the ratio of hair follicles in the anagen and telogen phases is normalized, and the intensity of hair loss is reduced. In addition, increasing the duration of the growth phase helps preserve active follicles. This leads to the restoration of healthy hair growth11.
Nurkrin products are manufactured in Europe (Denmark) and are designed for both women and men. Nurkrin products are widely used throughout the world and are highly valued by professionals in the field of hair restoration; since 2020 they have been introduced into the practice of Russian specialists.
Restoration of the natural hair growth cycle usually occurs within six months. In this regard, it is recommended to take a course of Nurkrin during the specified period.
Nucrine contains a unique complex, Marilex (fractionated fish extract with specific lectican proteoglycans). The complex contains components structurally associated with the hydrated matrix of the skin and hair follicles. It is rich in versican, decorin and syndecan, which are components of the dermal papilla and unique stimulators of hair follicle growth. Thus, Marilex contains components that stimulate the growth cycle of the hair follicle. In addition, Nucrine contains biotin and vitamin C, which provide nutrients to the hair roots.
A randomized placebo-controlled study demonstrated the superiority of Nucrine over placebo. Thus, after six months of therapy with Nurkrin, the amount of hair in patients increased by 35.7%, placebo - only by 1.5%15.
The results of clinical studies confirmed the bioavailability of proteoglycans included in Nurkrin. Based on the data obtained, it was concluded that the intake of specific proteoglycans included in Nucrin can lead to improved function and optimization of the life cycle of hair follicles16, 17.
“Proteoglycans are important components of the intercellular matrix of the dermis that regulate the hair growth cycle. Therefore, in case of hair loss, you can use the drug Nurkrin as a universal replacement therapy,” emphasized Professor E.A. Arabian in conclusion.
Beneficial properties of hyaluronic acid
Hyaluronic acid has a large number of beneficial properties, which we will try to list below:
- Hyaluronic acid is a gelling agent and it binds water very well, this is its main function. "Hyaluron" when forming gel-like liquids, binds 10,000 times the volume of moisture
- Due to its properties, hyaluronic acid will significantly accelerate the process of skin regeneration
- Hyaluronic acid helps prevent the destruction of collagen molecules, which serve as skin protectors.
- Hyaluronic acid has an anti-inflammatory effect
- Hyaluronic acid is a kind of special matter that has great moisturizing power
- Hyaluronic acid can collect and retain large amounts of moisture
- Hyaluronic acid allows you to deliver the necessary components and vitamins to the skin, which are so necessary for it, first of all, of course, to the skin of the face
- Hyaluronic acid is easily applied to the skin, leaving a thin film on the surface, thanks to which it collects moisture from the environment. Due to this, the moisture content in the skin not only does not decrease, but also increases, thereby preventing its dehydration.
At what age should you use hyaluronic acid?
In people's youth, the glycosaminoglycan gel is regularly renewed, thanks to which the skin can collect the life-giving moisture it so needs. Hyaluronic acid is also produced by fibroblasts in the amount it needs, thanks to which the skin of young people always looks so good and does not need additional care with hyaluronic acid.
After reaching 22-23 years of age, the amount of glycosaminoglycan gel produced by the human body begins to decrease, and the amount of hyaluronic acid produced decreases accordingly.
After 25 years, unfortunately, in addition to the fact that the glycan gel becomes smaller, its quality also decreases. This happens because the rate of renewal of the gel (containing hyaluronic acid) decreases and damaged collagen fibers begin to accumulate in the skin. Which in turn causes a decrease in the ability of the glycan gel to retain moisture, precisely due to a decrease in the amount of hyaluronic acid.
It is because of this that the skin of the face begins to look not fresh, becomes less elastic, and sometimes sagging due to the influence of gravity. Over time, wrinkles appear, “extra” skin, large folds near the nose and corners of the lips (nasolabial folds), wrinkles around the eyes, in the neck and, of course, décolleté.
Due to the constant influence of ultraviolet radiation on the skin, stress, improper care or poor nutrition, some of the water from this gel rises to the drier (superficial) areas of the skin, where it immediately evaporates.
It is important to know!
It is important to understand that the upper layers of the skin should not be dry, because as soon as they dry out, moisture to moisturize them begins to come from the deepest layers of the dermis, which in turn leads to even worse consequences associated with intense drying of the skin.
If overdrying of the facial skin begins, then unfortunately this leads primarily to rapid aging of the facial skin, and also contributes to the formation of wrinkles. In this regard, it remains extremely important for cosmetologists:
Provide facial skin (primarily) with continuous “water supply” and “water conservation”
Select the optimal set of effects on glycosaminoglycans so that they are regularly renewed and replenished with new cells